Organ Transplants: 3 Biomedical Advancements Transforming US Outcomes

Biomedical advancements are poised to revolutionize organ transplantation in the U.S., offering innovative solutions to combat organ shortages and improve patient outcomes through xenotransplantation, 3D bioprinting, and advanced organ regeneration techniques.
The landscape of healthcare is constantly evolving, and nowhere is this more evident than in the field of organ transplantation. For countless individuals awaiting life-saving procedures, the future holds immense promise. The Future of Organ Transplants: 3 Biomedical Advancements Set to Transform Patient Outcomes in the U.S. is not just a distant dream but a rapidly approaching reality, driven by groundbreaking scientific discoveries and technological innovations.
The pressing need for organ transplant innovation
The demand for organ transplants in the United States far outstrips the available supply. Thousands of patients remain on waiting lists, with many succumbing to their conditions before a suitable donor organ can be found. This critical shortage underscores the urgent need for revolutionary approaches to organ replacement and repair. Current transplantation methods, while life-saving, face significant hurdles ranging from donor compatibility to the lifelong need for immunosuppressive drugs.
Addressing these challenges requires a multi-faceted strategy, pushing the boundaries of what is currently possible in medicine. Researchers and clinicians are tirelessly working to develop solutions that can alleviate the burden on patients and healthcare systems alike. The advancements discussed here represent some of the most promising avenues for dramatically improving access to and success rates of organ transplants.
The current state of organ donation and transplantation
- Donor shortage: The primary challenge remains the severe scarcity of available organs, leading to long waiting lists and preventable deaths.
- Immunosuppression: Transplant recipients require lifelong medication to prevent organ rejection, which comes with significant side effects and health risks.
- Organ viability: The limited time organs can be preserved outside the body restricts transportation and potential utilization.
- Logistical complexities: Matching donors and recipients, and coordinating transplant surgeries, involves intricate logistical challenges.
These limitations highlight why groundbreaking biomedical advancements are not merely incremental improvements but essential paradigm shifts. The goal is to move beyond simply replacing organs to creating organs that are readily available, perfectly matched, and less likely to be rejected. This ambitious vision is slowly coming into focus thanks to rapid progress in several key areas of biomedical research.
Xenotransplantation: Bridging the donor gap with animal organs
Xenotransplantation, the process of transplanting organs or tissues from one species to another, has long been a subject of intense scientific interest and ethical debate. Recent breakthroughs, particularly involving genetically modified pigs, have brought this once-futuristic concept closer to clinical reality for human patients. The potential to use animal organs could fundamentally solve the critical organ shortage crisis.
Scientists have made significant strides in modifying pig organs to reduce the risk of hyperacute rejection, which occurs when the human immune system immediately attacks the animal organ. These modifications involve ‘knocking out’ genes responsible for producing sugars that trigger human immune responses and ‘knocking in’ human genes that help regulate the immune system. This genetic engineering is proving crucial in making pig organs more compatible with the human body.
Genetic engineering and immune compatibility
The primary hurdle in xenotransplantation has always been the vigorous immune response mounted by the recipient’s body. Early attempts often resulted in rapid organ rejection. However, modern genetic engineering techniques have allowed researchers to precisely alter the pig genome, making their organs more ‘human-like’ at a molecular level. This significantly reduces the likelihood of immediate rejection and could potentially lessen the need for aggressive immunosuppression.
- Gene editing tools: Technologies like CRISPR have enabled precise modifications to pig DNA, removing genes that trigger human immune attacks.
- Human gene insertion: Introducing human genes into pig cells helps the recipient’s immune system recognize the transplanted organ as less foreign.
- Immunosuppression reduction: Improved compatibility could lead to less potent immunosuppressive regimens for patients, reducing side effects.
While still in early clinical trials and facing regulatory scrutiny, the successful transplantation of genetically modified pig hearts and kidneys into human recipients (albeit in experimental settings) represents a monumental leap forward. These initial successes offer compelling evidence that xenotransplantation could one day provide an almost unlimited supply of organs, fundamentally transforming the lives of patients awaiting transplants.
3D bioprinting: Custom organs on demand
Imagine a future where organs are custom-made for each patient, perfectly matched to their unique biological profile. This is the promise of 3D bioprinting, an innovative technology that uses living cells and biomaterials (bio-inks) to construct functional tissues and organs layer by layer. This approach moves beyond simply replacing organs to creating personalized biological structures designed to integrate seamlessly with the patient’s body.
3D bioprinting leverages advanced imaging techniques to create detailed blueprints of a patient’s damaged organ. These blueprints then guide specialized bioprinters to deposit various cell types and structural components in precise arrangements, mimicking the complex architecture of natural organs. The goal is not just to create a scaffold but a living, functional organ capable of performing its intended physiological role.
Challenges and progress in bioprinting complex organs
While the concept is revolutionary, bioprinting a fully functional, complex organ like a heart or kidney presents formidable challenges. These organs require intricate vascular networks to supply nutrients and remove waste, a feat that is difficult to replicate with current bioprinting technologies. Additionally, ensuring the long-term viability and integration of bioprinted organs within the human body requires extensive research.
- Vascularization: Creating functional blood vessel networks within bioprinted organs is critical for their survival and function.
- Cell viability: Maintaining the health and function of printed cells during and after the bioprinting process is a significant hurdle.
- Structural integrity: Ensuring the bioprinted organ can withstand physiological stresses and integrate structurally with existing tissues.
Despite these challenges, significant progress has been made in bioprinting simpler tissues, such as skin, cartilage, and even rudimentary liver tissues. Researchers are continuously refining bio-ink formulations, developing more sophisticated bioprinters, and exploring novel methods to vascularize complex structures. The potential for creating on-demand, patient-specific organs represents a paradigm shift that could eliminate transplant waiting lists entirely.
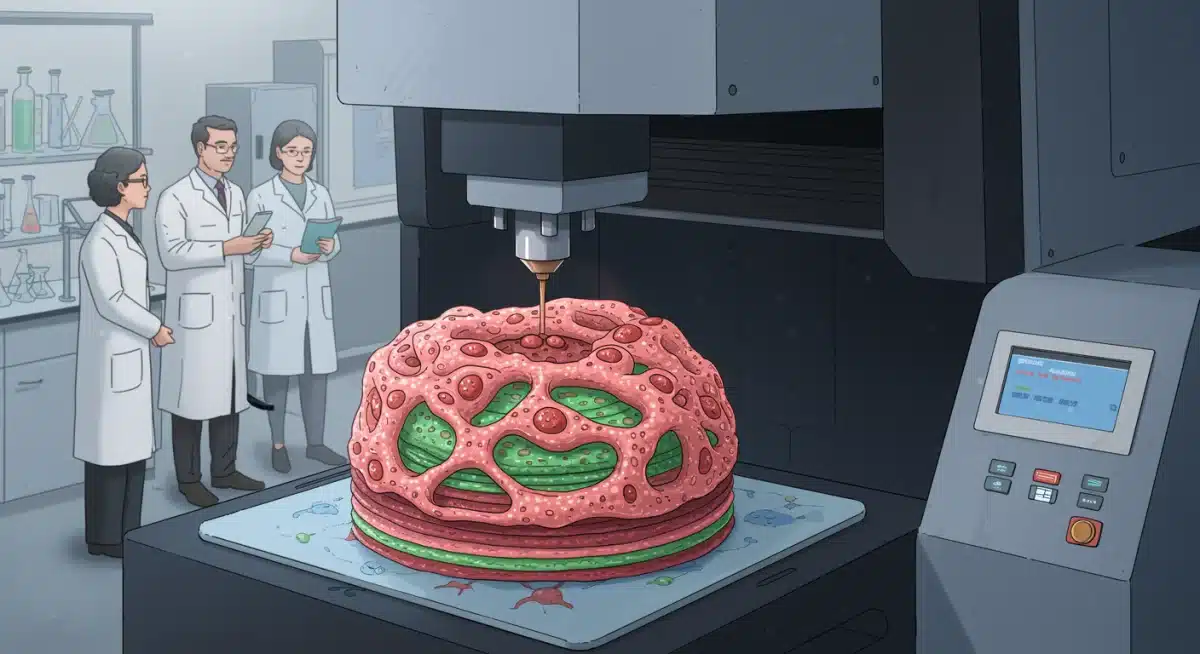
Organ regeneration and repair: Harnessing the body’s healing power
Instead of replacing a failing organ, what if we could regenerate or repair it using the body’s own intrinsic healing mechanisms? Organ regeneration and repair therapies represent another transformative frontier in organ transplantation. This approach focuses on stimulating endogenous repair processes, often involving stem cells or growth factors, to restore damaged organ function.
This field encompasses a broad range of strategies, from injecting stem cells directly into damaged organs to using biomaterial scaffolds that guide tissue regrowth. The ultimate aim is to harness the body’s remarkable capacity for self-healing, reducing the need for donor organs and minimizing the risks associated with transplantation, such as rejection and lifelong immunosuppression. The promise here is not just extending life, but restoring quality of life through fully functional, natural organs.
Stem cell therapies and gene editing for organ repair
Stem cells, with their unique ability to differentiate into various cell types, are central to many regenerative medicine strategies. By directing stem cells to differentiate into specific organ cells and integrating them into damaged tissue, scientists hope to rebuild and revitalize failing organs. Gene editing technologies also play a role, correcting genetic defects that lead to organ dysfunction or enhancing the regenerative capacity of native cells.
- Induced pluripotent stem cells (iPSCs): These cells, derived from a patient’s own body, can be reprogrammed to create any cell type, minimizing immune rejection risks.
- Growth factors and cytokines: Specific biological molecules can be used to stimulate the proliferation and differentiation of native stem cells within an organ.
- Decellularized scaffolds: Removing cells from donor organs leaves behind a natural extracellular matrix that can be reseeded with patient-specific cells to create a functional organ.
While still largely experimental, these regenerative approaches offer the tantalizing possibility of treating organ failure at its root, rather than merely managing its symptoms. The ability to repair or regrow a patient’s own organ would eliminate the need for an external donor, bypassing the complexities of compatibility and rejection. This area of research holds profound implications for chronic organ diseases and conditions currently requiring transplantation.
Ethical considerations and regulatory pathways
As these biomedical advancements progress from laboratory to clinic, they inevitably raise complex ethical questions and necessitate robust regulatory frameworks. Xenotransplantation, for instance, brings concerns about animal welfare, the potential for zoonotic disease transmission, and the moral implications of using animal life to sustain human life. Similarly, genetic modification and stem cell therapies require careful consideration of safety, long-term effects, and equitable access.
Regulatory bodies like the FDA in the U.S. play a crucial role in ensuring that these innovative therapies are safe and effective before they become widely available. This involves rigorous testing, transparent reporting, and ongoing monitoring. The ethical dilemmas surrounding these technologies demand open public discourse, involving patients, scientists, ethicists, and policymakers, to navigate the path forward responsibly.
Navigating the future of organ transplantation responsibly
Developing cutting-edge medical technologies is only one part of the equation; ensuring their responsible and equitable implementation is equally vital. The potential for these advancements to exacerbate existing healthcare disparities, if not managed carefully, is a significant concern. Access to these life-saving treatments must be considered from the outset, not as an afterthought.
- Bioethical committees: Essential for guiding research and clinical application, addressing moral and societal impacts.
- Transparent regulation: Clear and adaptable guidelines are needed to evaluate the safety and efficacy of novel therapies.
- Public engagement: Fostering informed public understanding and acceptance of new technologies is crucial.
- Equitable access: Strategies must be developed to ensure these advancements benefit all patients, regardless of socioeconomic status.
The journey from scientific discovery to widespread clinical application is long and fraught with challenges. However, the potential to save and improve countless lives provides a powerful impetus for overcoming these obstacles. Thoughtful consideration of ethical implications and a commitment to rigorous regulatory oversight will be paramount in realizing the full benefits of these revolutionary advancements.
The impact on patient outcomes and healthcare systems
The successful integration of xenotransplantation, 3D bioprinting, and organ regeneration into clinical practice promises to dramatically improve patient outcomes in the U.S. The most immediate and profound impact will be the reduction, and potentially elimination, of organ transplant waiting lists. This would mean fewer deaths while awaiting organs and a significantly higher chance of survival for those with end-stage organ failure.
Beyond merely increasing organ availability, these advancements could also lead to organs that are better tolerated by the recipient’s body, reducing the need for powerful immunosuppressive drugs and their associated side effects. This would translate to a higher quality of life post-transplant, fewer complications, and potentially longer organ survival. For healthcare systems, the implications are equally significant, potentially reducing the long-term costs associated with managing chronic organ failure and the extensive care required for transplant recipients.
Transforming chronic disease management
These innovations have the potential to shift the paradigm of chronic organ disease management from a focus on palliation and eventual replacement to one of restoration and cure. Imagine a world where kidney disease, heart failure, or liver cirrhosis no longer inevitably lead to a transplant waitlist, but rather to a regenerative therapy that restores the organ to full function. This would fundamentally alter the patient journey and the economic burden of these conditions.
- Reduced mortality: Fewer patients would die waiting for a donor organ.
- Improved quality of life: Less reliance on immunosuppressants and fewer post-transplant complications.
- Cost efficiencies: Potentially lower long-term healthcare costs by reducing chronic disease management and complex post-transplant care.
- Expanded eligibility: More patients who are currently deemed ineligible for traditional transplants due to age or comorbidities might become candidates for these new therapies.
The ripple effects of these advancements would extend far beyond individual patient care, influencing public health policies, medical training, and research priorities. The investment in these biomedical frontiers today will yield dividends in healthier populations and more sustainable healthcare systems tomorrow. The U.S. healthcare landscape stands on the precipice of a transformative era in organ transplantation, promising hope and healing to millions.
Future outlook and collaborative efforts
The trajectory of biomedical advancements in organ transplantation is undeniably upward, yet the path forward requires sustained effort, significant investment, and robust collaboration. The journey from initial discovery to widespread clinical application is lengthy, often spanning decades. However, the rapid pace of innovation, particularly in genetic engineering and personalized medicine, suggests that these timelines may accelerate.
Future success hinges on fostering interdisciplinary collaboration among scientists, clinicians, engineers, and ethicists. Public and private funding will be crucial to support the extensive research and development necessary. Furthermore, international cooperation can accelerate discoveries and ensure that these life-saving technologies are developed and deployed with global health equity in mind. The goal is not just to innovate, but to ensure these innovations reach those who need them most.
The role of technology and artificial intelligence
Emerging technologies, including artificial intelligence (AI) and machine learning, are also poised to play a pivotal role in accelerating progress. AI can analyze vast datasets to identify optimal donor-recipient matches, predict organ rejection, and even assist in the design of new bio-inks for 3D bioprinting. These digital tools will enhance the precision, efficiency, and safety of future transplant procedures.
- AI for matching: Advanced algorithms can improve the accuracy and speed of donor-recipient matching, reducing cold ischemia time.
- Predictive analytics: AI can forecast the likelihood of organ rejection or complications, allowing for proactive interventions.
- Robotics in surgery: Robotic assistance can enhance the precision and minimize invasiveness of transplant surgeries.
- Big data analysis: Leveraging large datasets to identify new therapeutic targets and optimize treatment protocols.
The convergence of biological science, engineering, and digital technology is creating an unprecedented era of innovation in organ transplantation. While challenges remain, the collective human ingenuity and dedication to improving health outcomes provide a strong foundation for optimism. The future of organ transplants in the U.S. is bright, promising a world where organ failure is no longer a death sentence but a treatable condition with high chances of full recovery and a return to a healthy life.
| Advancement | Brief Impact |
|---|---|
| Xenotransplantation | Offers a potential solution to the severe organ donor shortage by utilizing genetically modified animal organs. |
| 3D Bioprinting | Enables the creation of patient-specific organs and tissues, potentially eliminating rejection and waiting lists. |
| Organ Regeneration | Focuses on repairing or regrowing a patient’s own organs, reducing the need for external donors and immunosuppression. |
| Ethical & Regulatory Focus | Ensures responsible development and equitable access to these groundbreaking medical technologies. |
Frequently asked questions about organ transplant advancements
Xenotransplantation involves transplanting organs from animals, typically genetically modified pigs, into humans. By using animal donors, it aims to create a virtually unlimited supply of organs, drastically reducing the current severe shortage and saving countless lives on waiting lists.
3D bioprinting uses specialized printers to layer living cells (bio-inks) and biomaterials according to a digital blueprint of a patient’s organ. This process aims to construct functional, custom-made organs that perfectly match the recipient’s anatomy and physiology, minimizing rejection risk.
Yes, organ regeneration therapies focus on stimulating the body’s own healing mechanisms, often using stem cells or growth factors, to repair or regrow damaged organs. This approach could eliminate the need for donor organs and lifelong immunosuppression, offering a more natural restoration of function.
Key challenges include ensuring long-term organ function, preventing rejection (especially in xenotransplantation), creating complex vascular networks for bioprinted organs, and developing robust ethical and regulatory frameworks to ensure safety, efficacy, and equitable access for all patients.
These advancements promise to significantly improve patient quality of life by reducing waiting times, potentially eliminating the need for lifelong immunosuppressive drugs, and offering more durable and functional organ replacements. This translates to fewer complications and a healthier, more active life post-transplant.
Conclusion
The journey toward a future free from organ transplant waiting lists and the burden of chronic organ failure is long, yet the biomedical advancements in xenotransplantation, 3D bioprinting, and organ regeneration offer unprecedented hope. These innovations are not mere theoretical concepts but tangible breakthroughs poised to redefine healthcare in the U.S., promising a future where life-saving organs are more accessible, compatible, and effective. As research continues and ethical considerations are carefully navigated, the transformative potential for patient outcomes in the coming years is truly immense, ushering in an era of renewed health and vitality for countless individuals.